Human bone sarcoma cells express high levels of eIF4F components, particularly eIF4A2
We previously demonstrated that malignant peripheral nerve sheath tumor (MPNST), another aggressive type of sarcoma, overexpress components of the eIF4F complex20. To determine if this feature is characteristic of other types of sarcomas, we assessed the protein levels of both eIF4A isoforms 1 and 2 and eIF4E in four human and four canine osteosarcoma and two human Ewing sarcoma cell lines. Compared to MSCs, which have been postulated to be the cell-of-origin for bone sarcomas21, all of these human osteosarcoma and Ewing sarcoma cell lines overexpressed both eIF4A1 and 2 and eIF4E (Fig. 1A). In particular, the amounts of eIF4A2 were substantially higher in these sarcoma cells relative to MSCs. Similarly, all four dog osteosarcoma cell lines studied expressed high levels of eIF4A1/2 and eIF4E. These results indicate that osteosarcoma and Ewing sarcoma cells frequently overexpress eIF4F components.
Osteosarcoma and Ewing sarcoma cells express high levels of eIF4F components, which are important for tumor cell proliferation. (A) Western blots of lysates from human osteosarcoma and Ewing sarcoma (EWS) cell lines and canine osteosarcoma cell lines were probed for eIF4A1, eIF4A2, eIF4E, and GAPDH as a loading control. Human MSCs were used for comparison. (B) Genetic knockdown of eIF4A impairs osteosarcoma cell proliferation. Human osteosarcoma 143B, Saos2, and OS17 cells were infected with 10 MOI of lentiviral particles expressing a nontargeting shRNA (negative control) or shRNAs targeting eIF4A1 and/or eIF4A2. When the nontargeting-shRNA transduced wells reached confluence, cells were trypsinized and counted on a hemocytometer. The bar graphs depict the total number of cells, expressed as the mean ± SD of duplicate wells. The relative numbers of targeted shRNA-transduced cells, expressed as a percentage of nontargeting shRNA-transduced cells (defined as 100%), are listed above the bars (upper panels). Above the graphs are P-values relative to control cells, calculated using unpaired 2-tailed t-tests from duplicate wells from one (OS17) or two experiments (143B and Saos2). After counting, the harvested cells were lysed and analyzed by Western blots to detect eIF4A1 and eIF4A2 expression with GAPDH as a loading control. The relative band intensity of eIF4A1 or eIF4A2 was calculated by normalizing to that of GAPDH in each sample. These normalized values from biological replicates were averaged, and the mean changes in protein expression relative to the cells transduced with a nontargeting control shRNA estimated. The tables display the mean percent change in normalized levels of eIF4A1 and eIF4A2 protein after gene silencing (bottom panels).
Silencing of EIF4A1 or EIF4A2 reduces osteosarcoma cell growth
To examine whether eIF4A1/2 are important for osteosarcoma cell proliferation, we performed shRNA-mediated silencing in three human osteosarcoma cell lines. EIF4A1 knockdown in Saos2, 143B, and OS17 cells reduced the eIF4A1 protein levels by 78, 65, and 47%, respectively (Fig. 1B). Depletion of eIF4A1 in osteosarcoma cells caused an increase in eIF4A2 protein levels, particularly in 143B and OS17 cells. These increased eIF4A2 levels in EIF4A1 knockdown cells are likely due to compensatory transcriptional upregulation of EIF4A2 reported previously22. EIF4A2 knockdown in the same three osteosarcoma cell lines caused a 94–99% reduction of eIF4A2 protein levels, but eIF4A2 depletion was associated with increased eIF4A1 protein levels in OS17 and 143B cells. In addition, simultaneous knockdown of both EIF4A1 and EIF4A2 reduced the eIF4A1 and eIF4A2 protein levels in all three osteosarcoma cell lines, but their levels were not as low as those in cells with EIF4A1 or EIF4A2 knockdown alone. It is possible that this effect may also be due to similar compensatory transcriptional upregulation from each gene and warrants further investigation.
Importantly, silencing EIF4A1 or EIF4A2 slowed the growth of all three osteosarcoma cell lines, and the amount of growth inhibition correlated with the magnitude in the decrease of target protein levels (Fig. 1B). Co-silencing of both EIF4A1 and EIF4A2 also resulted in reduced cell growth in all three osteosarcoma cells, but the effect was slightly less than that by EIF4A1 silencing alone. Additionally, the effect of growth inhibition by combined EIF4A1 and EIF4A2 knockdown was less than that by EIF4A2 knockdown in 143B cells. These attenuated antiproliferative effects in double-knockdown cells may be due to less reduction in the levels of eIF4A1 and eIF4A2 proteins compared to those in single-knockdown cells. Nonetheless, the results indicate that both eIF4A1 and eIF4A2 are important for osteosarcoma cell proliferation.
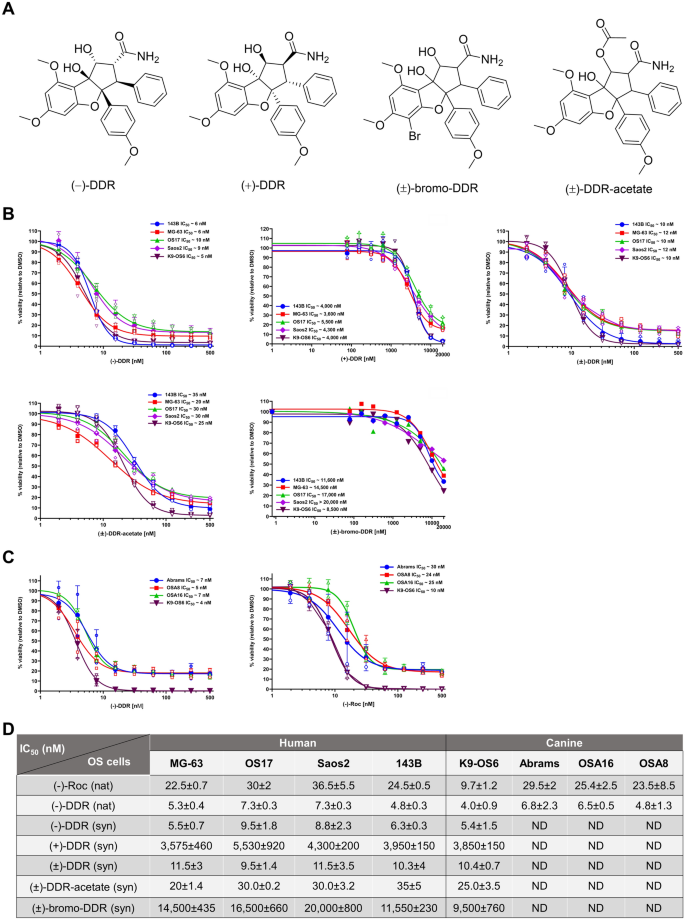
(−)-DDR, (±)-DDR, (−)-Roc, and the DDR derivative (±)-DDR-acetate potently inhibit proliferation of human and dog osteosarcoma cells
Studies have shown that bioactive rocaglates exert their anti-proliferative effects through eIF4A inhibition14,15. We also found that (−)-DDR and (−)-Roc potently suppress the growth of multiple types of sarcoma cells with (−)-DDR consistently showing two to threefold higher activity11. SAR analysis revealed that the C-8b hydroxy group on the cyclopenta[b]benzofuran core is essential for growth-inhibitory activity while modifying the C-2 and C-6 positions may enhance this activity. To expand SAR analysis, we synthesized racemic (±)-DDR and chirally separated racemic (±)-DDR into (−)-DDR and ( +)-DDR (Fig. 2A). As expected11, enantiopure (−)-DDR and (−)-Roc inhibited the growth of all four tested human osteosarcoma cell lines with IC50 values of 5–7 nM and 25–40 nM, respectively (Fig. 2B). The potent anti-proliferative activities of (−)-DDR and (−)-Roc were also observed in four dog osteosarcoma cell lines with IC50 values ranging from 4 to 7 nM for (−)-DDR and 10–30 nM for (−)-Roc (Fig. 2C). Intriguingly, when compared to (−)-DDR, racemic (±)-DDR was generally half as potent at reducing osteosarcoma cell proliferation (Fig. 2B). In contrast, the growth-inhibitory activity of ( +)-DDR in osteosarcoma cells was substantially reduced with IC50 values of about 3.6–5.5 µM. These findings support the importance of the (−) configuration of DDR in growth suppression.
(−)-Roc, (−)-DDR and racemic (±)-DDR and (±)-DDR-acetate potently suppress proliferation of human and dog osteosarcoma cells, while ( +)-DDR and (±)-bromo-DDR have greatly attenuated activity. (A) Chemical structures of (−)-DDR, ( +)-DDR, (±)-bromo-DDR, and (±)-DDR-acetate. (B) Actively-growing human osteosarcoma 143B, MG-63, OS17, and Saos2 and canine osteosarcoma K9-OS6 cells were treated with synthetic (−)-DDR, ( +)-DDR, (±)-DDR, (±)-DDR-acetate, or (±)-bromo-DDR as described in Methods. Cell proliferation was estimated as a percentage of the DMSO vehicle control designated as 100%. Graphs depict the means and SDs from 2 or 3 independent experiments. (C) Dog osteosarcoma Abrams, OSA8, OSA16, and K9-OS6 cells were treated with enantiopure (−)-Roc or (−)-DDR purified from Aglaia extracts, and cell proliferation was estimated as above. (D) Summary of the mean IC50 values for the indicated natural (nat) and synthetic (syn) rocaglates in various human and dog osteosarcoma (OS) cell lines. ND not determined.
To examine the effects of modifying the C1 and C5 positions, we synthesized (±)-DDR-acetate, which is acetylated at C1, and (±)-bromo-DDR, which is brominated at C5 of the A-ring (Supplementary Fig. S1). In various human and dog osteosarcoma cell lines tested, (±)-DDR-acetate had an IC50 value (20–35 nM) roughly 2–threefold higher than that of (±)-DDR (10–12 nM) (Fig. 2B,D). In contrast, (±)-bromo-DDR exhibited little growth-inhibitory activity, with an IC50 value ranging from 8.5 µM in the dog PDX-derived K9-OS6 cell line to > 20 µM in human Saos2 cells. These results indicate that acetylating the hydroxyl group at C1 of DDR is well-tolerated, while modifying the C5 position on A-ring aryl group substantially reduces antiproliferative activity.
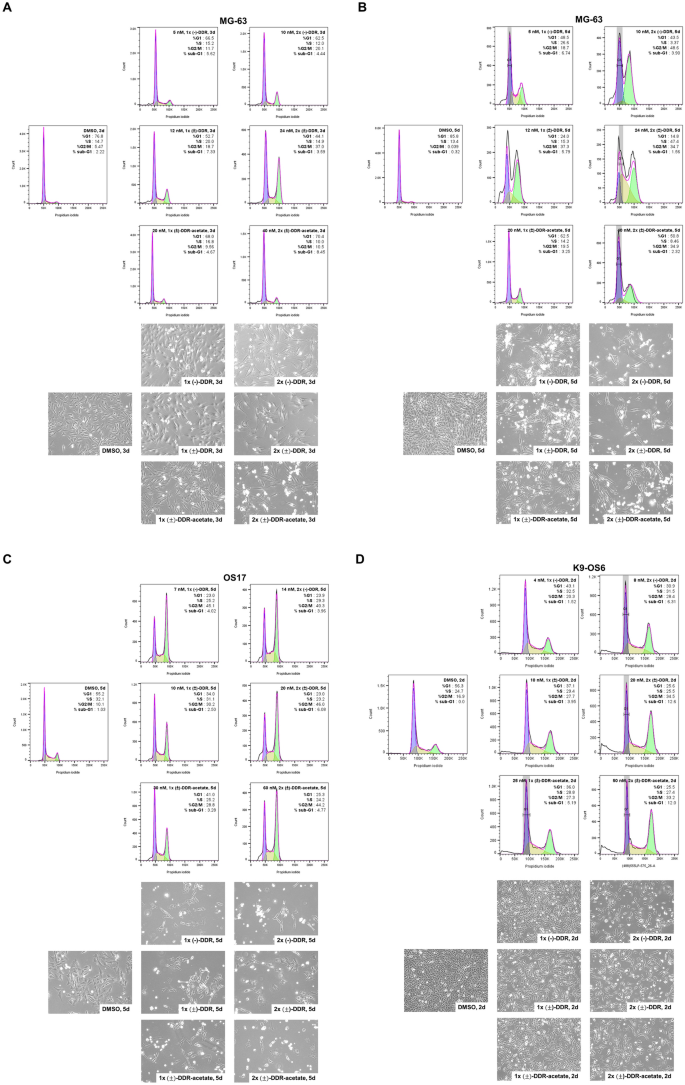
(±)-DDR-acetate, like (−)-DDR and (±)-DDR, induces G2/M arrest and apoptosis in human and canine osteosarcoma cells
To examine the mechanisms of growth inhibition, we first compared the effects of (±)-DDR-acetate, (±)-DDR, and (−)-DDR on cell cycle distribution of human and dog osteosarcoma cells. An increase in the G2/M population was observed in MG-63 cells treated with 1 × and 2 × IC50 concentrations of all three rocaglates for three or five days (Figs. 3A,B). Increased sub-G1 fractions were also observed in all three rocaglate-treated MG-63 cells, suggesting induction of cell death. Similarly, we detected prominent G2/M arrest and an increase in the sub-G1 fraction in OS17 cells treated with all three rocaglates (Fig. 3C). Like in human osteosarcoma cells, all three rocaglates also increased the G2/M and sub-G1 populations in K9-OS6 cells treated for two days (Fig. 3D). A similar increase in the G2/M and sub-G1 populations was observed in (−)-Roc-treated K9-OS6 cells (Supplementary Fig. S2). Intriguingly, human MSCs treated with (−)-DDR or (±)-DDR-acetate also showed increased G2/M fraction, albeit it was less than treated human and dog osteosarcoma cells (Supplementary Fig. S3).
(−)-DDR, (±)-DDR, and (±)-DDR-acetate induce G2/M arrest and cell death in human and dog osteosarcoma cells. MG-63 (A and B) and OS17 (C) human osteosarcoma cells were treated with 1 × and 2 × IC50 of (−)-DDR, (±)-DDR, or (±)-DDR-acetate for three (A) or five days (B and C). Treated cells were fixed, stained with propidium iodide, and analyzed for cell cycle distribution by flow cytometry. Shown are the cell cycle histograms with the % of cells in G1, S, and G2/M phases, as well as the sub-G1 fraction. Below the histograms are representative phase contrast micrographs of cells from each treatment prior to flow analysis. (D) K9-OS6 dog osteosarcoma cells were treated for 2 days with 1 × and 2 × IC50 of (−)-DDR, (±)-DDR, or (±)-DDR-acetate, followed by flow analysis. Cell cycle histograms were generated as described above.
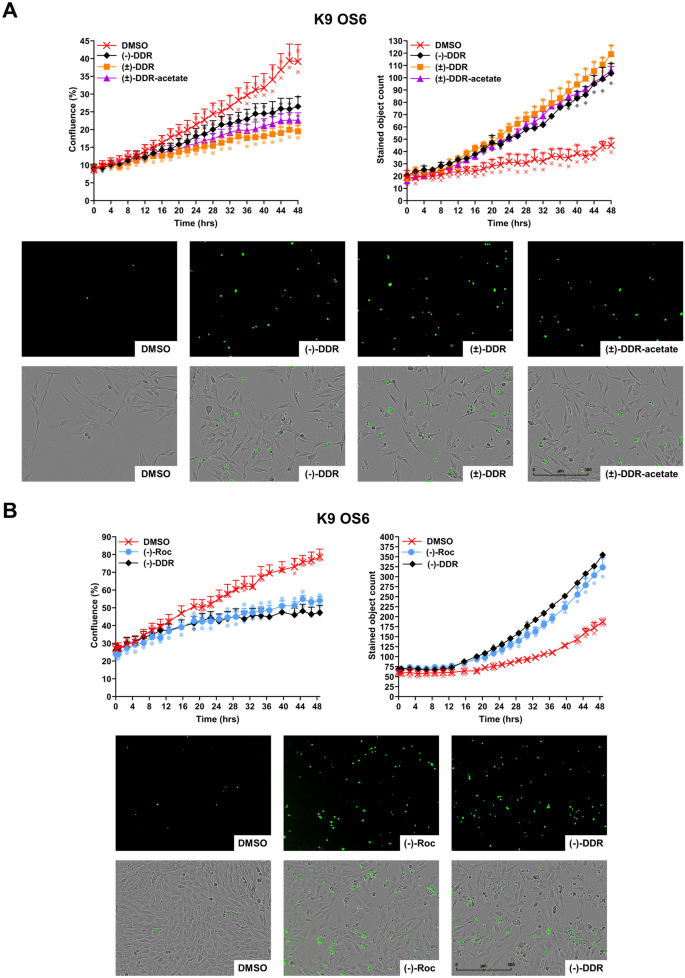
To determine whether induction of cell death by (±)-DDR-acetate, (±)-DDR, and (−)-DDR is due to apoptosis, we performed Incucyte live cell imaging combining with caspase-3/7 cleavage assay in K9-OS6 cells treated with each rocaglate. Growth inhibition was observed with concomitantly increased caspase-3/7 cleavage in cells treated with all three rocaglates, when compared to DMSO-treated controls (Fig. 4A). Significant increases in caspase cleavage were seen, starting from ~ 12 h of treatment and becoming more pronounced thereafter. Like (−)-DDR, (−)-Roc also inhibited the growth of K9-OS6 cells while simultaneously enhancing caspase-3/7 cleavage (Fig. 4B). These results were corroborated by flow cytometry analysis showing prominent increases in the G2/M and sub-G1 populations in (−)-DDR or (−)-Roc-treated cells (Supplementary Fig. S2).
(±)-DDR-acetate, (±)-DDR, (−)-DDR, and (−)-Roc inhibit proliferation of osteosarcoma cells by rapidly inducing caspase-3/7 cleavage, indicative of apoptosis. For dynamic quantitation of cell confluency and caspase-3/7 cleavage, K9-OS6 dog osteosarcoma cells were treated in duplicate with 2 × IC50 of (−)-DDR, (±)-DDR, or (±)-DDR-acetate and imaged in real-time on the Incucyte live-cell imaging system as described in Methods. Graphs depict the 48-h time course showing the percent confluency (left) and caspase-3/7 labeling (right) of treated cells (means ± SEMs) (A). Below the graphs are representative green fluorescence (indicating caspase-3/7 activation) and merged fluorescence and phase contrast micrographs of treated cells. Similarly, K9-OS6 cells were treated in duplicate with 2 × IC50 of (−)-Roc or (−)-DDR and evaluated over 2 days for confluency and caspase-3/7 activation using the Incucyte live-cell imaging (B).
Together, these results indicate that apoptosis induction contributes to the antiproliferative effects of (±)-DDR-acetate, (±)-DDR, (−)-DDR, and (−)-Roc in human and dog osteosarcoma cells.
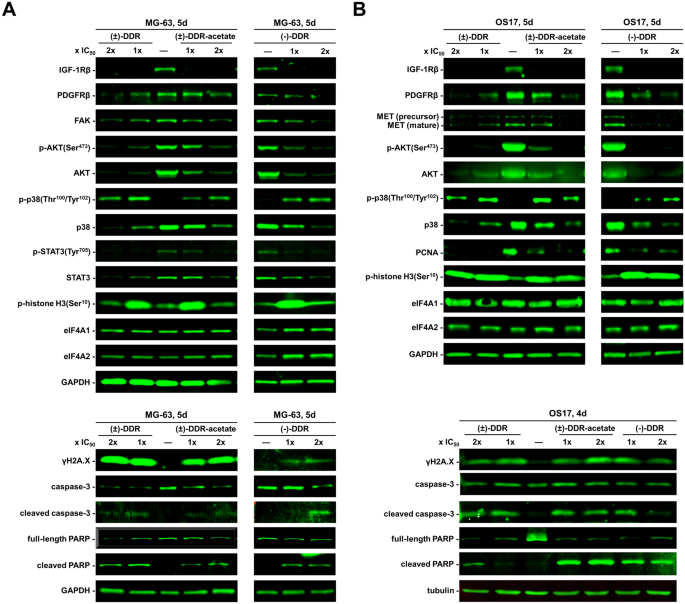
(±)-DDR-acetate also suppresses multiple signaling molecules important for osteosarcoma growth while activating cellular stress and DNA damage response pathways
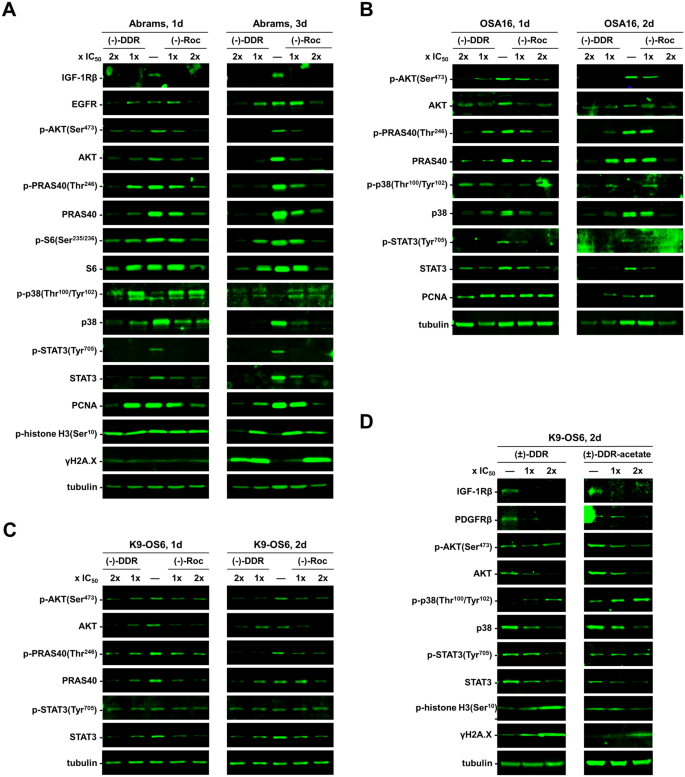
We previously showed that (−)-Roc and (−)-DDR inhibit the expression of several mitogenic kinases in sarcoma cells11. To examine whether the growth-inhibitory activity of (±)-DDR-acetate acted through similar molecular mechanisms, we performed Western blot analysis on human osteosarcoma cells treated with (±)-DDR-acetate, (±)-DDR, or (−)-DDR. All three rocaglates markedly diminished the levels of IGF-1R and AKT (total and phospho-AKT [p-AKT]) in both MG-63 and OS17 cells (Fig. 5). Also, reduced expression of PDGFRβ, p38 SAPK, FAK, and the transcription factor STAT3 (total and p-STAT3) was observed in treated MG-63 cells. In addition, these rocaglates decreased the MET levels in OS17 cells. Intriguingly, while p38 expression was diminished, phosphorylated p38 was notably enhanced, and the γH2A.X levels were elevated in treated MG-63 and OS17 cells, indicating activation of genotoxic stress signaling and DNA damage responses. In line with induction of G2/M arrest, we detected abundant p-histone H3, a G2/M marker, particularly in cells treated with 1 × IC50. Further, all three rocaglates increased cleavage of caspase-3 and PARP in both osteosarcoma cell lines, signifying enhanced apoptosis. Notably, the protein levels of housekeeping proteins GAPDH and tubulin, as well as eIF4A1, and eIF4A2 were unaffected by these rocaglate treatments. These results reinforce that eIF4A inhibition does not indiscriminately inhibit translation initiation but selectively affects transcripts encoding mitogenic proteins.
(−)-DDR, (±)-DDR, and (±)-DDR-acetate diminish the expression of multiple mitogenic kinases and concomitantly enhanced the levels of markers for cell stress, apoptosis, and DNA damage response in osteosarcoma cells. MG-63 (A) and OS17 (B) were treated 4 or 5 days (d) with 1 × or 2 × IC50 of (−)-DDR, (±)-DDR, or (±)-DDR-acetate. Equal amounts of protein from whole cell lysates were used in immunoblotting for the indicated proteins. GAPDH and tubulin were used as loading controls.
Similarly, we treated three dog osteosarcoma cell lines with (−)-Roc and (−)-DDR. Both rocaglates diminished the expression of IGF-1R, EGFR, total AKT, p-AKT, and its downstream targets PRAS40 and S6 in Abrams cells (Fig. 6A). Additionally, these rocaglates reduced the levels of total STAT3, p-STAT3, and PCNA. As in Abrams cells, (−)-Roc and (−)-DDR decreased the expression of AKT, p-AKT, its downstream substrate PRAS40, as well as STAT3 and p-STAT3 in OSA16 and K9-OS6 cells (Figs. 6B,C). Like in human osteosarcoma cells, (−)-Roc and (−)-DDR enhanced the phosphorylation of p38 in Abrams and OSA16 cells, despite decreases in total p38 levels (Figs. 6A,B). Also, we detected increased p-histone H3 and γH2A.X expression in treated Abrams cells (Fig. 6A). Further, both (±)-DDR-acetate and (±)-DDR reduced the expression of IGF-1R, PDGFRβ, AKT, and STAT3, while elevating phospho-p38 and γH2A.X levels in K9-OS6 cells (Fig. 6D).
Canine osteosarcoma cells treated with (−)-DDR, (±)-DDR, (±)-DDR-acetate, or (−)-Roc also exhibit reduced expression of mitogenic kinases but enhanced levels of markers for cell stress, apoptosis, and DNA damage response. Abrams (A), OSA16 (B), and K9-OS6 (C) dog osteosarcoma cells were treated with 1 × and 2 × IC50 of (−)-DDR or (−)-Roc for the indicated days (d). Equal amounts of protein lysates were immunoblotted and probed for the indicated signaling proteins with tubulin as the loading control. Also, immunoblots of protein lysates from K9-OS6 cells treated with 1 × and 2 × IC50 of (±)-DDR or (±)-DDR-acetate were performed and probed for various signaling molecules (D).
Overall, these data indicate that (±)-DDR-acetate, (±)-DDR, (−)-DDR, and (−)-Roc suppress human and dog osteosarcoma cell growth by decreasing the levels of key growth-promoting molecules and inducing cell stress and DNA damage responses, ultimately leading to apoptosis.
(−)-DDR and (−)-Roc potently suppress tumor growth in a canine osteosarcoma PDX model
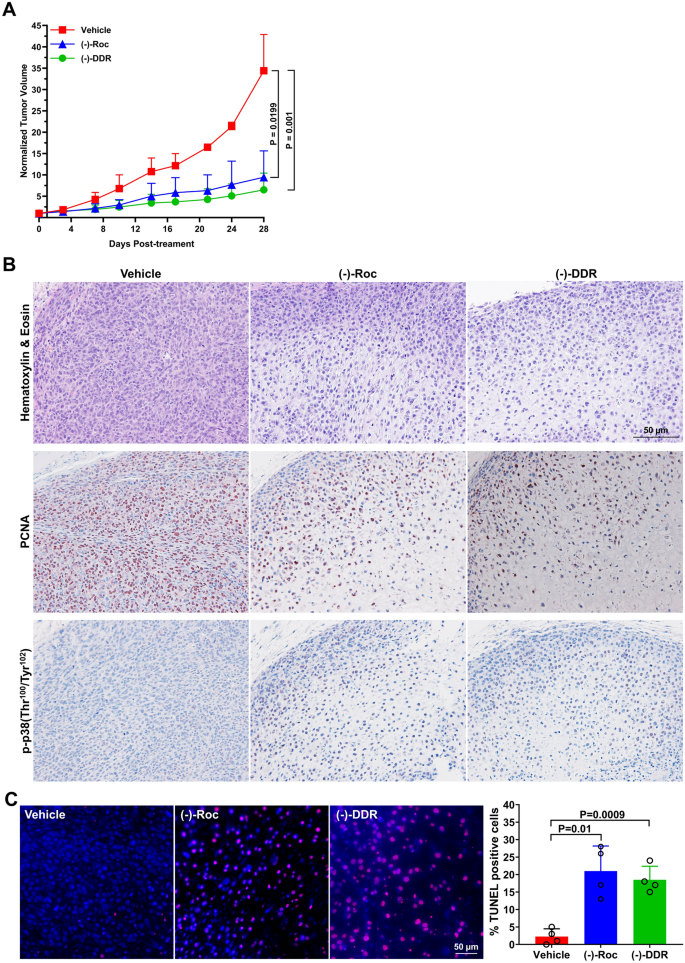
Since dogs frequently develop osteosarcomas with molecular signatures similar to human tumors6,7, we evaluated the in vivo efficacy of (−)-DDR and (−)-Roc using the OSU-K9-OS6 dog osteosarcoma PDX model. Compared to vehicle controls, (−)-DDR and (−)-Roc significantly suppressed tumor growth by ~ 81% and 73%, respectively, over four-week treatment (Fig. 7A and Supplementary Fig. S4). While vehicle-treated OSU-K9-OS6 PDXs exhibited sheet-like growth with high cellularity and active mitotic figures, (−)-DDR and (−)-Roc-treated tumors showed degenerative changes with substantially reduced cellularity, and immunohistochemistry (IHC) analysis confirmed decreased labeling of the proliferation marker PCNA and increased staining of phospho-p38 (Fig. 7B and Supplementary Fig. S5). Consistent with increased cleaved caspase-3/7 activities in vitro (Fig. 4B), TUNEL and cleaved caspase-3 staining demonstrated that both rocaglates greatly enhanced apoptosis in treated tumors (Figs. 7C and Supplementary Fig. S6). These results indicate that (−)-DDR and (−)-Roc also exhibit potent anti-tumor effects in dog osteosarcomas.
(−)-DDR and (−)-Roc effectively suppress tumor growth and promote apoptosis in a canine osteosarcoma PDX model. (A) NSG mice bearing actively-growing OSU-K9-OS6 PDXs were treated with (−)-DDR, (−)-Roc, or vehicle, and tumor volumes measured according to Methods. The mean fold-increase in normalized tumor volume over time was calculated by dividing each individual tumor volume to its value at the start of treatment set as 1. Shown are means ± SDs. The P-values are shown to the right for day 28 and were calculated using the cross-sectional functionality of the TumGrowth web application (https://kroemerlab.shinyapps.io/TumGrowth/) with generalized least squares fit by maximum likelihood model of log-transformed normalized tumor volumes. (B) Representative images of vehicle-, (−)-Roc-, and (−)-DDR-treated tumor sections stained with hematoxylin & eosin (top panel), or with the antibody against PCNA (middle panel) or p-p38 (bottom panel). (C) Tumor sections were also subjected to TUNEL staining and showed that DDR- and Roc-treated tumors had increased numbers of TUNEL-positive nuclei (red). DAPI stained nuclei in blue. The percentage of TUNEL-positive cells was estimated by counting ≥ 300 nuclei across 4 high-power fields in each treated tumor using the Cell Counter plugin of Fiji (https://fiji.sc). Bar graph shows the means and SDs of % TUNEL-labeled nuclei for each treated tumor. Above the graph are the P-values, calculated relative to the vehicle control using the unpaired two-tailed t-test with Welch’s correction.
(−)-DDR and (−)-Roc treatment upregulate genes and pathways linked to inflammatory responses, cell stress and death, and growth-related signaling
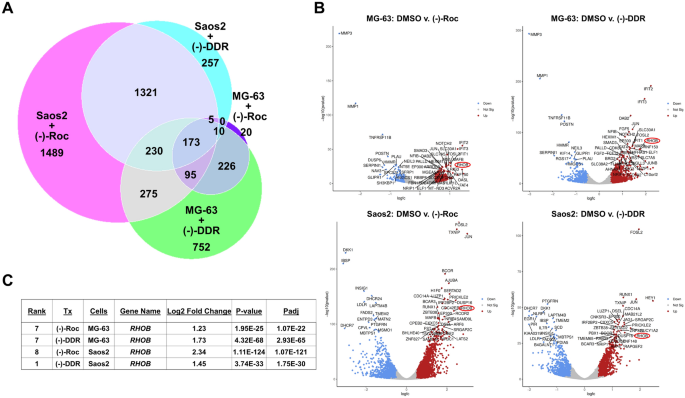
Prior studies demonstrate that the transcripts encoding transcription factors like MYC and JUN have long purine-rich 5’-UTRs and are sensitive to inhibition by (−)-Roc and related rocaglates23,24. As we found that (−)-DDR and (−)-Roc also decreased the STAT3 levels in osteosarcoma cells (Fig. 5), we investigated the effects of these rocaglates on the transcriptomes of MG-63 and Saos2 cells treated with (−)-DDR or (−)-Roc for one day by RNA-seq. Comparing the set of protein-coding DEGs in rocaglate vs. DMSO-treated cells, we identified 173 differentially-expressed transcripts in both rocaglate-treated MG-63 and Saos2 cells (Fig. 8A and Supplementary Table S1). Of these shared transcripts, 96% were similarly up- or downregulated by (−)-DDR and (−)-Roc treatment of MG-63 and Saos2 cells, including 121 upregulated (~ 70%) and 45 downregulated (~ 26%) DEGs (Supplementary Fig. S7). Of these 173 shared DEGs, many were also found among the top-50 protein-coding DEGs in both osteosarcoma cell lines treated with either (−)-DDR or (−)-Roc (compare Fig. 8B with Supplementary Table S1). These include upregulation of transcripts encoding the RhoB small GTPase, the dual-phosphatase DUSP16 which dephosphorylates and inactivates ERK and JNK, and the transcription factors JUN, FOSL2, MAFB, and HEY1; several of these proteins are important for osteosarcoma cell growth and differentiation.
RNA-seq analysis of (−)-DDR- or (−)-Roc-treated osteosarcoma cells identifies top common differentially expressed genes and pathways. MG-63 and Saos2 cells were treated 24 h with 1 × IC50 of (−)-DDR or (−)-Roc and analyzed by RNA sequencing. (A) Venn diagram of significant protein-coding DEGs (Padj ≤ 0.05; absolute log2-FC = 0.5) in treated osteosarcoma cells, showing that 173 DEGs are shared among all rocaglate-treated cells. (B) Volcano plots depicting the top-50 protein-coding DEGs identified in (−)-DDR- or (−)-Roc-treated MG-63 and Saos2 cells. Among these top DEGs, RHOB (circled in red) was detected in both osteosarcoma cell lines treated with either rocaglate. Blue = down-regulated; red = up-regulated; grey = non-significant. (C) Table summarizing the ranking of RHOB among the top-50 DEGs, the log2 fold-change, and the significance values in (−)-DDR- or (−)-Roc-treated MG-63 or Saos2 cells.
By GSEA, we identified 21 up- and 11 down-regulated pathways shared in (−)-DDR or (−)-Roc treated MG-63 and Saos2 cells (Supplementary Figs. S8 and S9 and Supplementary Table S2). The common top upregulated pathways included those involved in inflammatory responses and cytokine signaling (e.g. interferon-α and -γ responses and TNFα signaling), stress-related responses (e.g. UV response and hypoxia), G2/M checkpoint and apoptosis (e.g. p53 pathway, and mitotic spindle), and growth-associated NOTCH, WNT/β-catenin, and TGFβ signaling. The common top downregulated pathways were related to lipid metabolism (e.g. cholesterol homeostasis and fatty acid metabolism), E2F targets, mTORC1 signaling, glycolysis, and oxidative phosphorylation. These results are in line with decreased growth-promoting signaling and increased cell stress, DNA damage response, and apoptosis in (−)-DDR- or (−)-Roc-treated cells (Figs. 5 and 6).
Although (−)-DDR or (−)-Roc potently suppressed osteosarcoma growth, they did not eliminate all tumor cells (Fig. 7). Since RHOB was identified among the top ten upregulated DEGs in both MG-63 and Saos2 treated with either rocaglate (Figs. 8B,C), we tested whether increased RHOB expression provided any survival benefits. All four human osteosarcoma cell lines were tested with (−)-DDR and (−)-Roc in a dose-matrix combination with the Rho inhibitor Rhosin25. However, Rhosin alone had little or no growth-inhibitory activity even at 50 µM (Supplementary Fig. S10), and combining Rhosin with (−)-DDR or (−)-Roc did not give rise to enhanced growth inhibition according to Bliss synergy scores. These results suggest that RHOB upregulation in (−)-DDR or (−)-Roc treated osteosarcoma cells does not confer a survival benefit.